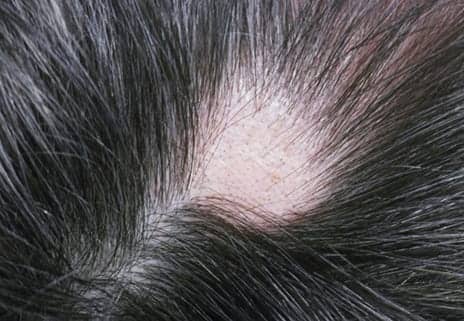
Cyclosporin is moderately effective for improving symptoms in patients with moderate to severe alopecia areata and may be considered as a second-line treatment for patients who fail to respond to corticosteroids, according to a study published in the Journal of the American Academy of Dermatology.
Currently, no efficacy trials have been performed on steroid-sparing agents used to treat alopecia areata, such as cyclosporin, methotrexate, and azathioprine. The investigators of this double-blind, randomized, placebo-controlled study sought to evaluate the short-term efficacy of cyclosporin vs placebo to treat adults with moderate to severe alopecia areata, according to a media release from Dermatology Advisor.
The single-center study included 32 participants aged 18 to 65 years and diagnosed with moderate to severe alopecia areata who were randomly assigned to receive 3 months of cyclosporin 4 mg/kg a day (n=16) or a matching placebo (n=16). Monthly assessments included physical examination, blood biochemistry, 2D and 3D photography, quality-of-life measures, concomitant medication use, and medication adherence. Efficacy was measured using Severity of Alopecia Tool (SALT) scores, as well as eyelash and eyebrow assessment scales.
After 3 months, 5 participants (31.3%) in the cyclosporin group achieved at least a 50% reduction in SALT scores compared with 1 participant (6.3%) in the placebo group (P =.07); however, this response rate was not deemed statistically significant. Overall, the cyclosporin group achieved a greater reduction in SALT score vs the placebo group (14.8% vs 2.3%; P =.23) and increased their nonvellus hair count (19.9 vs 1.8; P =.07).
Regarding eyelash and eyebrow assessments, 3 participants in the cyclosporin group achieved a 1-grade improvement in eyelash scales vs none in the placebo group (18.8% vs 0%; P =.07), and significantly, 5 participants in the cyclosporin group achieved a 1-grade improvement in eyebrow scales vs none in the placebo group (31.3% vs 0%; P =.02). No statistically significant difference in the rate of adverse events was observed between the groups, and no serious adverse events were reported.
Limitations to the study included a small sample size and single-institution trial design, which may prevent the generalizability of the study findings to a broader population. The short treatment duration may have prevented the ability to detect higher response rates given that agents used to treat alopecia areata typically have a delayed onset of action.
The results indicate that cyclosporin monotherapy was moderately effective at inducing hair regrowth in patients with moderate to severe alopecia areata, but the overall response rate did not reach statistical significance between the cyclosporin and placebo groups. The investigators suggest that these results may help guide clinicians in deciding on second-line treatment options for patients who fail to respond to systemic corticosteroids, the release continues.
[Source: Dermatology Advisor]