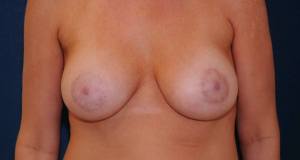
A female patient with adjustable implants.

The final result of the same patient following implant volume adjustment.
Internationally recognized for his expertise in plastic surgery, Hilton Becker, MD, FACS, of Boca Raton, Fla, has practiced plastic surgery since 1981. The veteran practitioner specializes in reconstructive and cosmetic surgery of the breasts, facial rejuvenation, and liposuction. He has lectured worldwide on his surgical techniques, published two books, contributed to numerous textbooks, and has published more than 50 articles in medical literature.
In addition, he has developed and patented several medical devices, including the Becker Adjustable Breast Implant, the Becker Dissector Liposuction (basket, twist, and greater) cannula, the BluntSeromaCath wound drainage system, and a line of skin care products.
The adjustable breast implant has been made commercially available as the Mentor Becker Implant, Spectrum® Adjustable Saline Implant, and the new Adjustable Gel Spectra®.
The Spectra’s unique feature is its volume-regulation system, which allows adjustments to be made during surgery and, if necessary, after surgery to attain ideal breast size. Traditionally, surgeons had to select from a vast array of differently sized prostheses to compensate for any asymmetry.
The Spectra’s adjustability facilitates achieving optimum size and correcting asymmetry. Size selection is facilitated when a mastopexy is performed along with breast augmentation, as well as the correction of congenital chest anomalies, such as Poland syndrome; in breast reconstruction following a mastectomy; correction of prior breast implant problems; and in patients that have abnormalities of the rib cage and pectoral muscles.
The Spectra is essentially a silicone gel implant with a small inner chamber that can be filled with variable amounts of saline or left completely empty. The inner chamber can be filled to give increased projection without palpable firmness. The detachable injection port allows adjustments to be made during or after surgery to attain ideal breast size, while causing minimal change in base diameter.
The Spectra is pending FDA approval in the United States, and is currently in studies at 30 sites.
Becker has also pioneered the subareolar (or “scar-less”) breast lift, which is used to treat ptosis of the breast.
PSP: Trace the history of breast reconstruction and the progress that has been made in recent years.
Hilton Becker, MD, FACS: When I was a general surgery resident, we did not do breast reconstructions. Patients with breast cancer in the past were treated with radical mastectomy. They presented late because they knew their fate would be a radical mastectomy.
Today, we see a totally different picture. We can do a one-stage immediate breast reconstruction at the time of the mastectomy, with virtually no visible scar. Now that patients know that they can have a one-stage breast reconstruction performed at the time of their mastectomy, they come in earlier with earlier disease and have lesser mastectomies saving more skin resulting in greatly improved results. What was originally a vicious cycle is now a positive cycle.
PSP: What exactly happened with the radical mastectomies in the last 30-some years?
Becker: Surgeons realized you did not need to do a radical mastectomy. They could do a modified radical mastectomy and the reoccurrence rate of the cancer was the same. The advent of the modified radical mastectomies enabled tension-free closure of the mastectomy site, and it became possible to perform tissue expansion breast reconstruction.
In the early 1980s, Chedomir Radovan came out with the first tissue expanders for breast reconstruction. The skin was stretched with a temporary expander, then the expander was replaced with a gel implant in a second procedure
I contacted Dr Radovan in the 1980s, and he sent me some of his expanders, and I started using them for breast reconstruction. They worked extremely well, and the patients loved the results. It was a very easy procedure compared to the flap procedures that had become available.
However, when the time came to remove the expanders, many patients did not want to take them out because they were quite happy with the results. I thought we could leave the expanders in. However, when left in for an extended period of time, wear and tear occurs at the junction of the filling tube and implant, resulting in leakage. I thought, if we could work out a way to detach the filling tube from the expander, we could convert the expander to an implant and leave it in as the definitive implant, enabling reconstruction to be performed in one stage.

A female patient postsurgery, using an adjustable implant and subareolar mastopexy (top), an adjustable implant being filled (bottom left), and the same patient after the procedure (bottom right).
PSP: Was this the basis for one of your implant products?
Becker: I took a single lumen saline implant to a medical corporation [Mentor Corp] and devised a special valve that would allow the filling tube and injection port to be detached. That was marketed as the Spectrum implant, and it became very popular with surgeons.
We then improved on this concept by creating a double lumen implant with silicone in the outer lumen. Initially, we had 25% silicone, then we went to the 50% silicone. The 50% version was called the Becker 50-50.That implant became the most popular implant for breast reconstruction manufactured by Mentor until we had the silicone moratorium in 1992. The implant remained available for reconstructive procedures.
When silicone was reapproved, the Becker 50-50, unfortunately, was not included at that time—I believed it would be grandfathered in. The FDA, however, decided it should be taken it off the market and undergo the same study that the silicone gel implant underwent.
The studies have now been completed, and we are eagerly awaiting reapproval.
PSP: Tell us about your latest implant, the Spectra.
Becker: While working with the Becker 25 and 50-50, I realized that the more gel the better, especially for the cosmetic patient. There was also a need for an implant with higher projection and an implant that could help with asymmetry and implant size selection.
The obvious solution was to have an implant that was 100% gel yet had the ability to be adjusted to increase size and projection. The Spectra that was developed looks and behaves so much like a silicone gel implant that you cannot tell the difference. However, it has a small inner lumen that can be left completely empty. With the Becker 50-50, you cannot leave it empty without any saline in the inner lumen because it is half the volume of the implant. With the adjustable gel implant, the saline volume can be 0%, 10%, 20%, or 30%. The Spectra has been launched in most other countries in the world, but it is not yet available in the US.
The Spectra implant has very wide indications. One of the major problems we see is breast asymmetry or patient dissatisfaction with size. With the Spectra, you can correct asymmetry by making fine adjustments postoperatively. At one of the recent meetings, it was reported that asymmetry after a breast augmentation is so common that we should warn our patients that to some degree, asymmetry is inevitable. However, that is not true. With Spectra, you can change the volume of the surgery and you can correct for asymmetry.
We are seeing many patients that are getting prophylactic mastectomies with thicker skin flaps, so we can put in implants with larger volumes of gel. We also have the ability to correct asymmetry. Patients who have had breast reconstruction with tissue expanders, upon replacing the expander with a gel implant, invariably, the volume is incorrect. If the tissue expander is replaced with a Spectra, we are able to fine-tune the volume to obtain the correct size. This is particularly important in revision cases when a patient has had surgeries resulting in scarring, asymmetry, or infections. Where factors are unpredictable, it is very difficult to know what size implant to use.
The implant solves so many problems and helps in so many areas. It gives the breast implant a completely new dimension. I hope that surgeons in the US will have it available to them within a couple of years.
PSP: What do you see happening in terms of the next step in reconstruction and implants?
Becker: I think the next step is a whole change in the concept of the surface of the breast implant. The trend is moving back to smooth implants, even for anatomical implants. One of the manufacturers already has a “smooth” anatomical implant on the market. In the US, most surgeons prefer using smooth-surfaced implants for augmentation and reconstruction. I think that is going to happen in Europe and South America, as well.
The next era in reconstruction, I believe, is going to be in the ability to place implants above the muscle. Placing an implant under the muscle is not anatomically correct. Abnormal muscle contraction can be bothersome. The ability to place the implant above the muscle will be facilitated by surgeons leaving thicker flaps and the use of new meshes both biological and synthetic. We will also be able to thicken flaps with fat injections and stem cells.
PSP: On another front, most of what I hear about the new bio-mesh products has been positive.
Becker: It is not all positive. There are a few negatives with biologicals. The problem is that the industry is ahead of the research. There must be 10 different biologicals, and we do not know which one is the best. Which one has the lowest incidence of infections, seroma, and breakdown? Which ones vascularize the most rapidly? And pricing is still an issueThere is also a need for better synthetic mesh. What we have available to us now are too thick for breast reconstruction. We need thinner, finer measures for breast reconstruction.
PSP: Can they be made stronger but not as thick?
Becker: The problem is that they tend to be too rigid. When the mesh is too thin, it becomes too elastic. There needs to be some way to be able to make the mesh convert from a rigid to elastic. There are different breakdowns in properties of the mesh. We are working on having it break down in a controlled way, so that it can remain strong and nonelastic, initially, and then become more elastic at a later date and also offer a scaffold for tissue in-growth.
PSP: Is a product such as the internal bra something that will be a temporary solution, or will it be integrated with this sort of bio-mesh technology?
Becker: We are using acellular dermis to compensate for a shortage of muscle when an implant is placed under the muscle. When an implant is placed above the muscle, a large sheet of mesh is needed to strengthen the coverage over the implant. A mesh bag containing the implant with the ideal properties would be best.
PSP: Are there other technologies that show promise in solving these kinds of problems?
Becker: What they are looking at now is a synthetic scaffold having similar characteristics to dermis. Synthetic scaffolds can function in a very similar fashion to acellular dermal scaffolds.
PSP: Do you have any comment on the inevitable FDA approval and widespread use of gummy bear-type implants?
Becker: They are in common use now. They are excellent. I use them all the time.
The Becker 50-50 and the Spectra contain cohesive gel (gummy bear). We are looking forward to these being released by the FDA.
Jeffrey Frentzen is the editor of PSP. Shae Waddell is a contributing writer for PSP. She can be reached at [email protected].