Breast augmentation surgery is one of the most common procedures in the plastic surgery world, yet postoperative pain is sometimes underestimated by surgeons as not being an important part of the patient’s experience.
Peter T. Pacik, MD, FACS, has extensive training in a wide range of cosmetic and reconstructive surgical procedures. Pacik currently limits his Manchester, NH-based practice to cosmetic surgery.
He has written many publications dealing with breast enlargement and has also lectured on this topic at plastic surgery conferences.
Pacik is nationally recognized for his work on postoperative pain control after breast augmentation. In an article published in the Aesthetic Surgery Journal,1 Pacik describes the continued use of pain-control catheters to help control postoperative pain in augmentation mammaplasty patients. Data analysis of a study conducted by Pacik revealed pain to the sternum, sides of the chest, below the clavicle, and back, in addition to the expected breast pain.
Pacik’s research revealed that older women had less pain and that frightened patients had more pain—and the latter used narcotics for a longer period of time. Otherwise, there were no statistical differences as related to the size of the breast implants, intraoperative expansion, handedness, duration of surgery, and different techniques of dissection to create the implant pocket.
In addition to noting the continued safety in using pain-control catheters, Pacik determined that instilling a long-acting anesthetic into the augmentation mammaplasty breast pocket was as effective in controlling postop pain as narcotics.
Pacik is a member of several organizations, including the American Society of Plastic and Reconstructive Surgeons, the American Society for Aesthetic Plastic Surgery, the International Society of Clinical Plastic Surgeons, the New England Society of Plastic and Reconstructive Surgeons, and the New Hampshire Medical Society. In addition, Pacik is a fellow of the American College of Surgeons.
PSP: Please describe your approach or approaches to pain management in breast surgery.
Pacik: Surgical procedures of the breast result in primarily localized pain for the patient, so I’ve tried to find ways to deliver localized pain medication in an effort to avoid narcotizing the entire patient. To that end, I began working with pain-control catheters almost 15 years ago.
 |
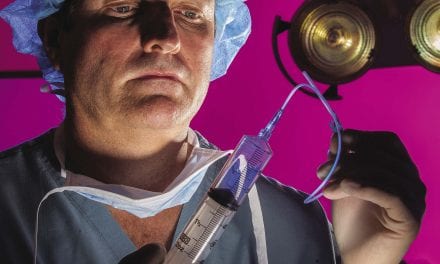
| Figure 1. Bloody aspirate is visible about 45 minutes after office instillation of 20 mL of bupivacaine into each catheter. The extension tubing is placed between the syringe and the catheter. |
Ideally, pain management begins in the operating room. The use of a gentle, methodical dissection and perhaps a bit more time in the operating room has not been shown to correlate to an increase in postoperative pain.
We inject Sensorcaine before the incision in breast surgery is even made to help control pain once the patient awakes in recovery, and ice packs also provide some relief immediately, postop.
In some cases, narcotics are warranted as part of recovery, but in many cases Ibuprofen will suffice. We also install indwelling catheters to each breast and use Sensorcaine in each catheter. This method has proven to provide another 6 to 8 hours of comfort in most patients. We also recommend four more ibuprofen once the patient is discharged and arrives home, as well as rest and sleep.
The more normal the patient’s activities, the better. For example, we encourage patients to get out of bed, shower, and even blow-dry their hair so that the arms get some movement. This helps avoid stiffness of the pectoralis major (the muscle dissected during the procedure). The brain translates this stiffness as pain, so if patients put their arms through a full range of activities (getting dressed, putting on makeup, and even going out for dinner), the muscles return to normal faster and the patient is able to return to normal faster.
We offer three doses of Sensorcaine available during the next 24 hours. Each installation is 20 cc of 0.25% Sensorcaine with 1:400,000 epinephrine. If needed, patients are able to use narcotics, ibuprofen, Tylenol, and muscle relaxants. This gives them the ability to use some form of medication every 2 hours as needed, and gives them control over their pain-management routine.
Overall, having a patient return as quickly as possible to the normal routine of showering, washing hair, blow-drying hair, etc, helps to maintain activity of the chest muscles. This routine can be repeated as many times during the day as the patient desires. The catheters we use are cleaned with normal soap and water, and are washed during normal bathing activities. When chest muscles (or any muscle) undergoes surgical trauma, the brain translates the resulting stiffness as pain. Movement lessens the stiffness and, therefore, the perception of pain postop.
PSP: How well do your patients adjust to using continuous flow devices or the pump?
Pacik: In my experience, both continuous flow devices and the on-demand pump work equally well. However, most patients opt for the on-demand pump for purely economical reasons: The continuous flow device can cost as much as $200, and is removed after 24 hours. The on-demand pump is much more economical and doesn’t have to be removed.
Additionally, the patient has control over their pain medication and can administer the drugs as needed, whereas the continuous flow device dispenses medication continuously, whether the patient is experiencing pain or not.
In my experience working with more than 800 patients using an on-demand pump, all have consistently expressed extremely high levels of satisfaction. The ability to control the amount of pain medication and its timing gives postop patients control, and this has been received very well in patient populations.
PSP: Has the rest of the medical world, specifically plastic and cosmetic surgeons, adopted similar practices to pain management following surgery? In other words, is there a need for new education for surgeons on this issue?
Pacik: Surgeons who use pain-control catheters love them. The ones who don’t use them find excuses about why not and quote articles that don’t advocate them, perhaps even if the articles are based on studies that aren’t sound from a research perspective.
One article—which was against the use of pain-control catheters—used study results and data from a study that only analyzed a total patient population of 11. Another group studied patients who, in some cases, may have undergone more than one procedure. In both examples, the articles used data to support their conclusions that didn’t result from good research techniques.
The study I conducted overwhelmingly showed that pain-control catheters do work. In fact, our data showed that pain-control catheters are just as effective as narcotics (including Vicodin and Percocet), and our study confirms that pain-control catheters are statistically just as safe and effective.
PSP: How should a physician, in your estimation, first communicate with the patient about these devices and approaches?
 |
| Figure 2. Closeup of the Luer lock end of the catheter without the attached extension tubing in a patient 1 day after her augmentation mammaplasty. |
Pacik: It is always of the utmost importance to discuss pain and pain management during the initial consultation. Postop pain should be expected. It is a normal part of every surgery. We discuss every available option with patients, including pain-control catheters and medications. Our goal is to keep them comfortable after the surgery. Every patient is unique, and every person has a different pain threshold.
It is of the utmost importance to inform patients that discomfort is to be expected, but if they follow our instruction and administer pain medication appropriately and resume normal muscle movement as quickly as possible, then recovery time is shortened.
Not only is pain management critical, but so is “fear management.” Setting patient expectations is a critical piece of the communication process, and directly impacts satisfaction with the overall procedure.
PSP: What are the issues and concerns with pain-management techniques?
Pacik: Good hygiene is the most important piece—keeping the catheters clean and washing hands before touching the catheters will keep infection from becoming an issue. Normal bathing with appropriate cleansers will alleviate concerns about possible infection, but we always encourage our patients to be aware and to use precautions to avoid infections. Once patients understand that infection can mean the loss of an implant, then they do their part to keep the area clean.
Dosing is another consideration. I prescribe 20 cc of pain medication for each breast to be administered every 6 to 8 hours, and then the patient can use other analgesics in-between, if needed.
PSP: What about pain pumps?
Pacik: We offer all patients a choice between self-administration versus pain pumps, as I mentioned earlier. Cost is usually a big factor, and we have a published study that compares the pain pump to self-administration where both systems work equally well. The choice is entirely up to the patient, but it is our job as caregivers to provide enough information that the patient can make an educated choice.
PSP: What do you see as the future of pain management? How quickly does the technology advance in this area?
Pacik: The future of pain management has more to do with medical practitioners than with technology. It is becoming more evident that one of the keys to pain management is asking patients the right questions during recovery. Physicians would do well to consider pain one of the basic vital signs (pulse, blood pressure, heart rate, etc) during postoperative evaluations. Current practice doesn’t include recording data related to the amount and the location of pain experienced by patients in postoperative evaluations.
There are also multiple ancillary techniques that can help control pain and at least lessen its duration. As I said before, if patients get up and move around, and in breast surgeries move their arms doing normal everyday activities, then patient feedback is very positive.
That being said, every patient is different. I have spent significant time looking at correlations between different variables. For example, does the size of the implant indicate more pain for the patient? If there is increased expansion necessary during the surgery, does that necessarily indicate more pain? Our data shows that neither of these variables necessarily means more pain for the patient. Pain is completely variable, just as every patient is different.
PSP: Postmastectomy pain appears to be a common problem, in which interventional therapy seems to be more common than a preventative strategy. While any surgery patient may experience pain upon waking, the suffering of mastectomy patients often lasts for years without relief. For how long do you track the patient postoperatively?
Pacik: Postmastectomy pain that lasts for an extended period of time may indicate a patient with chronic pain issues. Our approach to pain management is intended for postsurgical patients to manage their discomfort for a limited time period.
However, I did have one patient with a specific issue that we were able to resolve. This patient developed chronic pain involving the left lateral aspect of her breast after a mastectomy. We tried padding the bras to take the pressure off the area, but pain persisted and did not abate. I suspected fourth intercostal nerve entrapment, so I tried injecting the area with Sensorcaine, but this gave only temporary relief.
After 1 year, I explored the area and identified the fourth intercostal nerve within the capsule. I rotated fat flaps from above and below the nerve to help cushion it, and this helped long term, allowing her to be pain free. This is the only time I have encountered a patient with long-lasting pain issues postsurgery.
Schae Kane is a contributing writer for PSP. She can be reached at [email protected].
REFERENCE
- Pacik PT, Nelson CE, Werner C. Pain control in augmentation mammaplasty using indwelling catheters in 687 consecutive patients: Data analysis. Aesthet Surg J. 2008;28:631-641.
USING BOTOX TO TREAT VAGINISMUS AND RAYNAUD’S DISEASE
Botox has become a powerful tool for the treatment and cure of vaginismus, according to Peter T. Pacik, MD, FACS. Vaginismus, which is characterized by extreme fear of any form of vaginal penetration, is similar to any phobia in that it can be disabling because of its severity. The fear of vaginal penetration results in involuntary and uncontrolled spasm of the vaginal muscles. In the more severe cases of vaginismus, intercourse is impossible, and even pelvic exams may be out of the question.
Health care providers, be they nurses or physicians, are often unfamiliar with vaginismus and therefore even the diagnosis of vaginismus is often delayed for many years. Most of my patients made their own diagnosis by researching the Internet. Once the diagnosis of vaginismus is made, most physicians still don’t know what to do. They need to make some referral. The first choice is often a psychologist or sex therapist. However, “You can’t talk a severe vaginismus patient into a successful outcome. They listen politely and make no progress. Much time, money, effort, and emotion is wasted,” Pacik says.
“If vaginismus is indicated, then classifying its severity is very important in ensuring appropriate therapy,” he continues. “Severe grades of vaginismus are usually unable to respond to conservative therapy, and patients may spend years moving from therapist to therapist. Spasms related to vaginismus are an uncontrolled involuntary reflex due to an extreme fear (phobia) of pain resulting from penetration.”
For suffering patients, a complete history is extremely important, he adds, since the initial indications can be seen in the teen years—many patients are even unable to use tampons.
“Later in life, patients with vaginismus are typically unable to undergo a gynecological exam,” he explains. “Approximately 80% of these patients have some form of positive psychosexual history, such as sexual molestation, strict upbringing, being told by friends about ‘first time’ pain, bleeding, tearing, or rough gynecological exams.”
The treatment is more than simply injecting Botox for vaginismus. “I perform a comprehensive program of injecting Botox under anesthesia, progressively dilating the vagina at the same time, and leaving a dilator in place that the patient wakes up with in the recovery room,” Pacik says. The dilator is coated with a topical anesthetic.
In addition, a series of long-acting local anesthesia injections (bupivacaine) are administered so that the patient is numb in the vagina when she wakes up. Therefore, she does not feel the dilator in place at first. She must sleep with a dilator that is comfortable. The patient is seen again each day for the next 2 to 3 days to continue working with the dilators. All this takes place before the Botox has had a chance to work. Once the Botox becomes effective—between 2 to 10 days—the process gets easier.
“Essentially, Botox relaxes vaginal muscles so that intercourse can be experienced and, in many cases, enjoyed without pain,” Pacik says. “Ultimately, relief from vaginismus has a profound impact on relationships. To date, we have documented a 90% cure rate with intercourse achieved as early as 5 days to 2 to 3 months post-treatment with Botox.”
RAYNAUD’S DISEASE
Pacik has also explained the use of Botox as a treatment for advanced Raynaud’s disease. “When symptoms include extreme contraction of muscles or numbness/coolness in extremities, Botox can be an extremely effective remedy,” he says.
Raynaud’s disease causes the small arteries that supply blood to the skin to narrow, which limits blood circulation. This condition is more common in women than in men, and in people who live in colder climates.
Indications include the following:
- Cold fingers and toes;
- Color changes in the skin in response to cold or stress; and,
- Numb, prickly feeling, or stinging pain upon warming or relief of stress.
“Skin usually turns white during the initial stages of a Raynaud’s attack. Areas can progress to a blue coloration and may feel cold and numb,” Pacik notes. “In many cases, pain is so intense that patients sometimes cry out for amputation, just to make the pain go away.
“Botox has the same effect in this instance as it does when treating vaginismus,” he adds. “It relaxes the muscles so that blood can flow through arteries to the skin. In severe cases, this treatment can avoid amputation due to dead tissue and can help heal ulcerations.”
—SK